
The Patient Voice Database empowers you to shape better treatments by connecting you the Research — safely, and always on your terms.
You will be requested to answer some questions from our Artificial Intelligence, based on the thousands of trials that are currently recruiting.

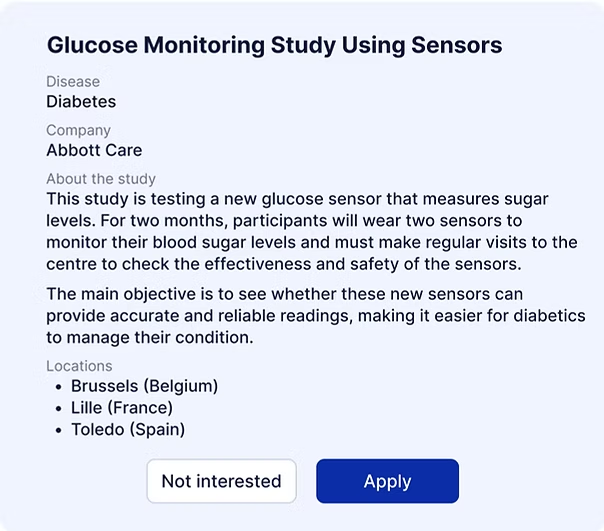
Based on your responses, Curewiki will generate a list of trials that match your profile and preferences.
You decide whether you want to volunteer and be put in touch with the principal investigator.
Registering does not commit you to anything more than receiving trial suggestions.

Answer a few questions — no medical files needed. It is private and secure.

We show you only the opportunities that fit your needs and no one contacts you without consent.

Interested? Connect. Not ready? Ignore. It's always up to you.
"Everyone has the right to take part in medical research. That's why we're opening up access to all clinical trials that are recruiting in Europe. What's more, our independence maximises your chances of finding the right trial for your needs."
Jean-Sébastien, Founder of Curewiki
Curewiki is a European startup (12 people across the Netherlands, Belgium and Spain) founded in 2022 and financed with public and private funds.

A childhood cancer survivor, Jean-Sebastien wants everyone to receive a treatment that helps them heal. He leads the company.

A former doctor who used to grapple with the difficulty of finding clinical trials for patients and acquaintances, Edwin set up a medical research company before joining Curewiki. He is in charge of commercial relations.

With 15 years in fintech, health tech, and retail, Mieke leads strategies that connect patients to clinical trials with innovation and measurable impact.

With 10+ years in tech and digital business, Olga drives market expansion and builds impactful partnerships to scale growth.

DON'T STOP BELIEVING.
Any questions? See hereafter if we already have an answer for you.